Photo Credit: Laurent Ballesta
By Nicole Rivard
I’ve always appreciated horseshoe crabs for their compelling appearance and because they evoke nostalgia.
They transport me back to renting summer cottages along the shore of Clinton, Connecticut, and Misquamicut, Rhode Island, with my family, grandmother, aunt and cousins.
Back then, I didn’t know I should also cherish them for protecting my loved ones and me from exposure to potentially lethal contaminants known as endotoxins when we got our childhood immunizations, flu shots or any intravenous drugs.
That’s because the test pharmaceutical companies use to detect toxic substances in injectable medicines and implants—including insulin, heart stents, hip replacement systems and more recently, the Covid shot— is made from the blue blood of the horseshoe crab. Their blood is used to produce a substance called limulus amoebocyte lysate, or LAL.
Today, there are five FDA-licensed LAL producers along the East Coast.
However, it’s no longer necessary to bleed the species dry and to extinction. There is a synthetic compound that can be used to test intravenous drugs, but unfortunately the U.S. has lagged behind other countries in recognizing it as viable.
That is, until now.
Last August, U.S. Pharmacopeia (USP), a nonprofit that sets scientific standards for the pharmaceutical
industry, proposed a new standard for endotoxin testing that would use non-animal products—the reagents rFC and rCR. In January, Friends of Animals submitted comments to USP in support of the proposal.
If the standard is approved, it would be available for early adoption Nov. 1 and official as of May 1, 2025.
“The time to end the cruel bleeding of horseshoe crabs and employ safe and effective alternatives to LAL is long overdue,” said Jennifer Best, director of Friends of Animals Wildlife Law Program. “We commend USP’s efforts to switch to animal-free methods and materials and urge USP to promptly approve animal-free alternatives to LAL, such as rFC and rCR. Horseshoe crabs are declining and at risk of extinction if current practices continue.”
Alternatives for horseshoe crab blood have been available for around two decades. These synthetic
alternatives were invented and patented in the 1990s by researchers at the National University of Singapore. The European Pharmacopeia has endorsed the use of rFC since 2019 and noted several advantages, including its reproducibility, quality, sustainability and specificity, as have regulating bodies in Japan and China.
“USP’s proposed standards are a vital step towards safe and ethical alternatives. More than 140 countries rely on USP’s standards,” Best said.
Catalyst for change
In 2018, a study co-authored by Revive & Restore, an organization promoting the incorporation of
biotechnologies into standard conservation practice, confirmed the efficacy of the synthetic alternative. It also found that the biomedical industry could achieve a 90 percent reduction in the use of reagents derived from horseshoe crabs by using the synthetic alternative—a huge step toward protecting horseshoe crab populations.
“Our research allowed us to engage media with the deeper story—why rFC was languishing on the
market—since it was proven to be equally efficacious,” said Ryan Phelan, co-founder and executive director. “I think the media campaign we launched was the right pressure at the right time. A lot of voices were raised and amplified.”
The study also found that the synthetic is more cost-effective and more widely available than ever. While there are up-front costs for switching, in terms of new equipment and software, and an investment in time, the synthetic reagents are less expensive, so that helps offset costs.
“More importantly, switching to rFC improves the safety, efficiency and viability of endotoxin testing, which is hugely valuable for pharmaceutical companies,” explained Kika Tuff, communications director, Revive & Restore. “Horseshoe crabs are a finite resource, whereas the synthetic can be manufactured at scale. Companies can leverage their good work to meet sustainability goals and attract new customers. Ultimately, pharmaceutical companies gain more than they spend in converting.”
Just ask pharma giant Eli Lilly. The lack of standards provided by USP over the years didn’t stop the company from successfully converting 80 percent of their testing of medicines from LAL to rFC. They now use it in all eight of their injectable manufacturing facilities and for all their new injectable medicines.
“Eli Lilly has had to work with international colleagues and largely forge this path for American
pharmaceuticals,” Tuff said. “They had to verify over and over that their testing was safe and effective. They have demonstrated that it’s possible, as well as financially viable in the long-term. They have faced all kinds of financial, regulatory and technical obstacles but overcame them—driven by their commitment to horseshoe crab conservation.”
It’s time for the rest of the industry to follow suit.
Bleeding labs, which drain horseshoe crabs of about 30 percent of their blood and turn that blood into LAL, collected 637,029 horseshoe crabs in 2019, 30 percent more than they took the year before, according to The Washington Post. While the crabs are returned to the water, at least 15 percent—or 95,554—die. Some research puts that mortality figure as high as 30 percent.
Horseshoe crabs have a brain, eyes, mouth, heart and nervous system—all protected by a large plate of
armor. The species has been a vital part of the environment—shorebirds like the endangered red knot feed on their eggs to survive—since before the dinosaurs, dating back approximately 300 million years ago.
Unfortunately USP’s proposal doesn’t mandate that pharmaceutical companies use the synthetic alternative or prohibit using horseshoe crab blood alternative. But FoA recognizes it as an important development in the effort to restore and save Atlantic horseshoe crabs.
“This is what incremental change looks like and it should be celebrated,” said Priscilla Feral, president of
Friends of Animals. “But even if this proposal is accepted, FoA understands it needs to be combined with other protections, such as state and federal bans on killing horseshoe crabs. Our efforts on those fronts will continue until the unethical and unnecessary bleeding and killing of these ancient mariners for medical research or for bait for eel and snails is stopped everywhere in the United States.”
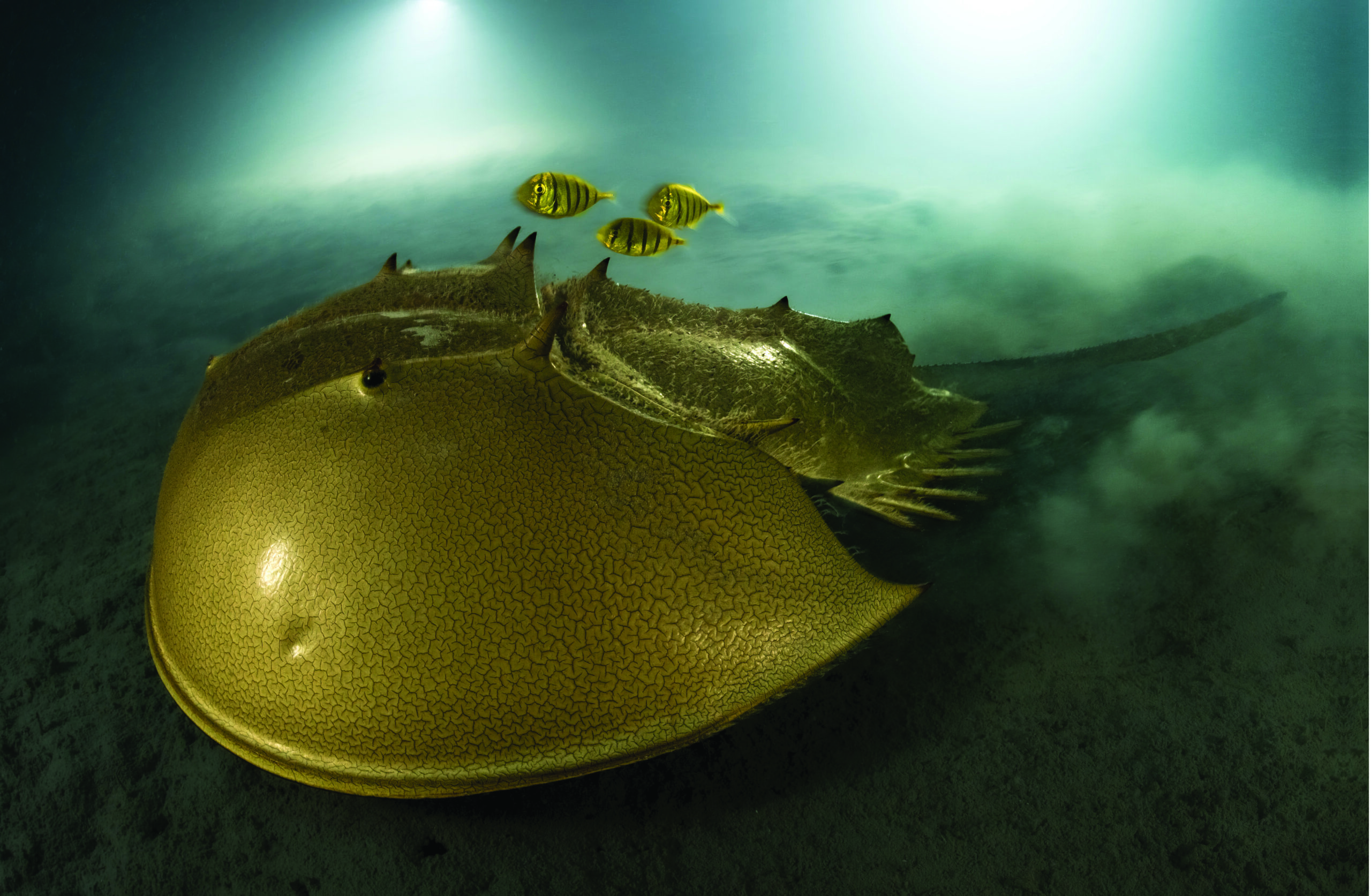
About the photography
The photograph on pages 8-9 of a tri-spine horseshoe crab, accompanied by a trio of golden trevallies, was taken by French underwater photographer and marine biologist Laurent Ballesta in the protected waters of Pangatalan Island in the Philippines. Looking almost like an extraterrestrial spaceship, the gilded carapace of the arthropod gliding through the dark waters amazed judges so much Ballesta was awarded Wildlife Photographer of the Year 2023 by the London Museum of History.
“To see a horseshoe crab so vibrantly alive in its natural habitat, in such a hauntingly beautiful way, was
astonishing,” said Kathy Moran, chair of the jury. “We are looking at an ancient species, highly endangered…..This photo is luminescent.’
The tri-spine horseshoe crab has survived for more than 100 million years but are now under threat from
habitat destruction and fishing—with hundreds of thousands of horseshoe crabs killed annually to be used as bait for other species and for scientific research.